By Dr Fiona McMillan
Researchers at The University of Queensland Diamantina Institute and The University of Sydney Centenary Institute have developed a 3D model of a melanoma tumour that enables them to visualise the growth states of cancer cells in real-time. In a paper published in the journal Pigment Cell and Melanoma Research, they describe how they have used cutting-edge imaging technology to develop a 3D model that precisely measures cell cycles as a melanoma tumour grows.
This provides remarkable visual evidence that melanoma tumours can evade therapy by arresting the growth of some cells and reveals new information about the nature of spreading tumour cells.
“In solid cancers, including melanoma, not all tumour cells are the same,” says Associate Professor Nikolas Haass of UQDI, which is based at the Translational Research Institute in Brisbane.
A single tumour is a diverse ecosystem of cells that not only have a wide variety of functions, but are at different stages of their growth. He is particularly interested in how this affects metastatic melanoma, which is highly treatment resistant.
“Melanomas are composed of zones of fast growing cells next to zones of dormant cells and zones where cells invade into the surrounding tissues,” he says. “This tumour heterogeneity is thought to contribute to cancer drug resistance, as cells in different growth states respond differently to drugs.”
A subpopulation of tumour cells is able to enter a state called G1-arrest where they stop growing and dividing. Not only do most chemotherapies work by causing DNA damage in dividing cells, which triggers the cells’ own self-destruct mechanisms, but modern targeted therapies also often rely on cell division. Thus, most current treatments have little effect on cells in stasis.
Moreover not much is known about the growth state of cells that break off from the main tumour. It was proposed that they also enter G1 arrest while in transit to other tissues.
Assoc. Professor Haass believes that understanding growth dynamics of all the individual cells in a tumour could reveal a great deal more about the underlying biology of melanoma.
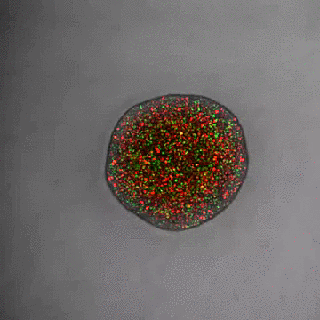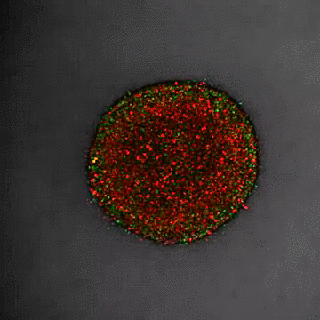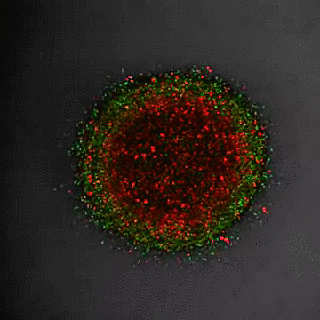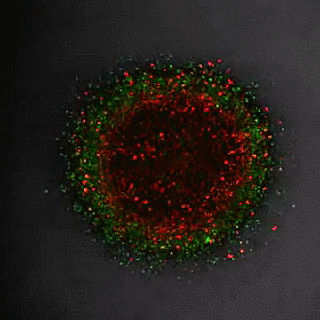
Using fluorescent markers they observed tumour growth using time-lapse microscopy. Cells in G1-phase are red and stay red if arrested; growing zones go through the cell cycle and exhibit red, yellow and green cells representing all cell cycle phases. Cells in the centre of the tumour rapidly become dormant (red), while cells in the periphery and invading cells continue to divide (changing colours).
“We found that some melanoma cells are dormant in conditions with low oxygen and nutrients or in the presence of new anti-melanoma drugs, but quickly recommence growth and invasion after re-exposure to a more favourable environment,” says Haass.
“Importantly, dormant melanoma cells are able to survive for long periods and may be responsible for recurrence of the tumour.”
When the cells began to grow again, the researchers re-treated the tumours. They found that the majority of the cells were still sensitive to the drugs, suggesting that the persistence of the cancer was due more to ‘sleeping’ cells re-entering the growth cycle rather than due to genetic changes causing drug resistance.
“We therefore propose that targeting of dormant melanoma cells is necessary for decreasing the likelihood of recurrence,” says Haass.
He explains that the research also revealed important new information about migrating tumour cells: they are not in G1-arrest.
“We are the first to show in real-time that melanoma cells grow actively during invasion into surrounding tissues.”
These findings challenge the idea that melanoma cells can either invade or proliferate. In fact, they can do both. Moreover, the results suggest that cells in stasis are poor invaders.
Haass is looking for the molecular triggers that turn G1 arrest on and off. He is particularly interested in discovering which pathways are turned on in G1-arrested cells and targeting those for potential future therapy.
MEDIA: Kate Templeman on 0409 916 801 or k.templeman@uq.edu.au