Jones Group - Molecular genetics
About
Dr Jones’ research is focused on developing novel therapies that specifically target tumour cells by exploiting their genetic and metabolic vulnerabilities. Dr Jones's team uses cutting edge molecular, cell biology and genomics approaches to understand how processes such as DNA replication, DNA repair and cellular metabolism are misregulated in cancer. The ultimate goal of this research is to translate this knowledge into improved patient outcomes through chemotherapies strategies that are both selectively toxic to cancer cells and harness the immune system for long lasting protection.
Investigating DDK-dependent regulation of stalled replication forks
DNA replication is the fundamental mechanism of genetic inheritance. In cancer cells, replication is corrupted and replication forks frequently stall and collapse causing DNA damage and copying errors that drive tumorigenesis. As a result, cancer cells are heavily dependent on the pathways that protect and repair stalled replication forks. Disrupting these mechanisms can be selectively toxic to cancer cells. A key player in the regulation of DNA replication and repair is DDK (Dbf4-dependent kinase also known as Cdc7). DDK is frequently overexpressed in cancer, but its role during DNA replication and the repair of stalled forks has not been well characterised. Our team is using chemical genetic approaches to selectively target DDK and gain valuable insights into its requirements and molecular targets.
Applying long-read sequencing technology to understand telomere repair and DNA replication
Our team is appling long-read DNA sequencing technology to dissect the spatial and temporal regulation of DNA replication and understand repair pathways at telomeres. By implementing long-read sequencing approaches in these research areas, we aim to advance our structural and functional understanding of the human genome.
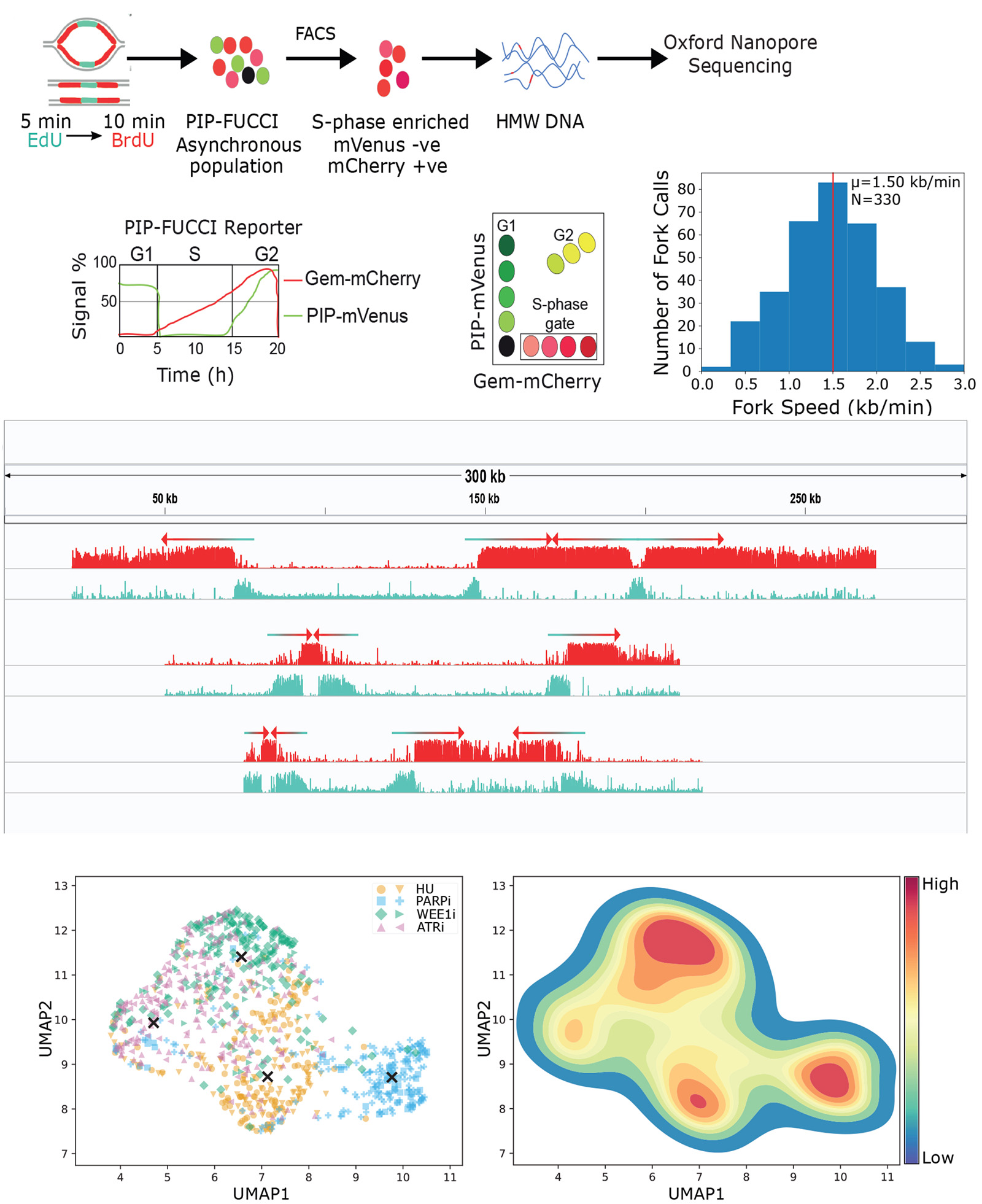
Grants
ARC-DP (2021-2023) - Decoding the spatiotemporal control of DNA replication and repair
ASSC – Reversing the “Warburg effect”: Targeting metabolic reprogramming and DNA repair to enhance immunogenic cell death in melanoma
- GIH - Applying long-read sequencing technology to understand telomere repair and DNA replication
Partners
- Prof Paul Clarke (UQDI)
- Prof Kum Kum Khanna (QIMRB)
- Prof Prasad Jallepalli (MSKCC)
- Dr Julia Pagan (UQ-SBMS)
- Subash Rai (UQ-GIH)
- Valentine Murigneu (UQ-GIH)
- Michael Boemo (Cambridge University)


